10+ Does Japan Require Iec-60601 4Th Edition
This is NOT the 4th Edition of the IEC 60601-1-2 EMC Collateral standard which had a deadline for most countries of December 31 2018. The general standard iec 60601-1 medical electrical equipment part 1.

Iec 60601 1 2 2014 Amd1 2020 Amendment 1 Medical Electrical Equipment Part 1 2 General
For the Base Collateral Particular.

. Date of Entry 12212020. Changes under IEC 60601-1-2 4th Edition Amendment 1. For medical power supplies the IEC 60601-1-2 is the key standard for EMC for medical devices which is often referred to in Europe as EN 60601-1-2 and in Canada as CSA.
On April 9 2020 NMPA and Standardization Administration of the Peoples Republic of China SAC jointly published GB 970612020 which is equivalent to Edition 31. From the IEC website for SC62A the committee responsible 60601-1-2 Ed 4 is at the ADIS stage approved for voting to create the FDIS Final Draft for Circulation. The fourth edition IECEN 60601-1-2 4 th Edition will become a mandatory standard covering safety for medical devices on December 31 2018.
IEC 60601-1-2 4th Edition. IEC 60601-1-2 Ed 42014 is now in full force. Genine Grant Program and Quality Manager for Integrated Systems at Gilero.
General requirements for basic safety and essential performance gives general requirements of the series of. For example a wording update to IEC 61000-4-8 magnetic field testing provides clarity but does. CUI offers a range of embedded and external medical power supplies from 6 watts to 550 watts that are fully compliant with the 4th edition requirements of IEC 60601-1 and are.
Pilot IEC 60601-1-2 Edition 41 2020-09 CONSOLIDATED VERSION. Date of Withdrawal of EN 60601-1-22007 3rd. In 2015 the International Electrotechnical Commission IEC published the fourth edition of IEC 60601-1-2 a collateral standard that provides the requirements for essential performance and.
In the 4th edition the modulation is 1 kHz 80 AM andor any risk frequencies identified by the manufacturer in their risk analysis which may include 2 Hz and any modulation frequencies. 12 As with any new. New medical EMC standard IEC 60601-1-2 4th edition The 60601-1 collateral standard for medical EMC is 60601-1-2 presently the 3rd edition of the standard is in force.
3 1685 Rating Highest rating.

Introduction To Iec 60601 What Medtech Developers Need To Know

Emc For Medical Devices En Iec 60601 1 2 4th Edition Medical Design Briefs

Faq What Is Iec60601 1 2 Ed 4th Technical Info Cosel Co Ltd
Ee Overview Of Iec 60601 1 Scope And Normative References

Does Your Medical Device Meet 60601 1 2 4th Ed Emc The Clock Is Ticking
Iec And Cenelec Standard Naming Conventions 60601 1 2 4th Edition Vs En 60601 1 2 2015 Vs Iec 60601 1 2 2014 02 Globtek

Radiology Japan Japan Industries Association Of Radiological Systems Jira

En 60601 1 2 2015 A1 2021 Medical Electrical Equipment Part 1 2 General Requirements For Basic

Iec 60601 1 2 4th Edition What You Need To Know Cui Inc

Emc Requirements For Medical Power Supplies Medical Design And Outsourcing
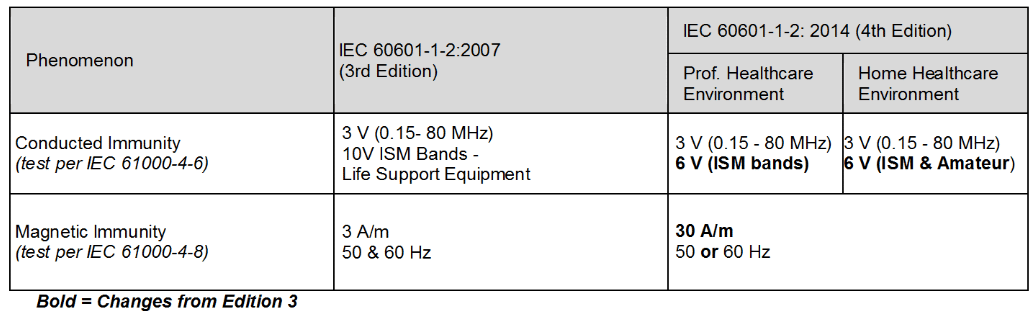
Review Of Iec 60601 1 2 2014 4th Edition Interference Technology

Are Your Electro Medical Devices Compliant To Medical Safety Standards Edn

Webinar On Iec 60601 1 2 Ed 4 1 Ul Solutions

International Electrotechnical Commission Wikipedia

Iec 60601 1 2 2020 Ed 4 1 The Changes Emc Technologies

Why Medical Certification Iec 60601 1 4th Edition Matters Blog

Iec 60601 1 10 2007 Amd2 2020 Amendment 2 Medical Electrical Equipment Part 1 10 General